 A team of researchers from Rice University have taken lithium ion batteries to their next step of evolution. Their aim to develop three-dimensional batteries is now closer to reality by the implementation of a PMMA polymer (aka plexiglas) encasing the arrays of nickel-tin nanowires than make up the anode.
A team of researchers from Rice University have taken lithium ion batteries to their next step of evolution. Their aim to develop three-dimensional batteries is now closer to reality by the implementation of a PMMA polymer (aka plexiglas) encasing the arrays of nickel-tin nanowires than make up the anode.
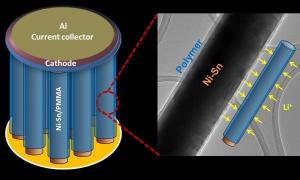
“In a battery, you have two electrodes separated by a thick barrier,” said Pulickel Ajayan, professor in mechanical engineering and materials science and of chemistry. “The challenge is to bring everything into close proximity so this electrochemistry becomes much more efficient.”
They found a way to safely coat single nanowires with a smooth layer of a PMMA-based gel electrolyte. While this gel allows lithium ions to pass through, it also insulates the nanowires that elongate during charging from the other electrode.
“We wanted to figure out how the proposed 3-D designs of batteries can be built from the nanoscale up,” said Sanketh Gowda, a graduate student in Ajayan’s lab. “By increasing the height of the nanowires, we can increase the amount of energy stored while keeping the lithium ion diffusion distance constant.”
Although the three-dimensional concept is not new, this is an improvement to it. For the first time, the Rice researchers put the PMMA coating over long distances on nanowires, and are now testing how the technology could be implemented on more powerful nanowire batteries.
The process builds upon the lab’s previous research to build coaxial nanowire cables that was reported in Nano Letters last year. In the new work, the researchers grew 10-micron-long nanowires via electrodeposition in the pores of an anodized alumina template. They then widened the pores with a simple chemical etching technique and drop-coated PMMA onto the array to give the nanowires an even casing from top to bottom. A chemical wash removed the template.
They have built one-centimeter square microbatteries that hold more energy and that charge faster than planar batteries of the same electrode length. “By going to 3-D, we’re able to deliver more energy in the same footprint,” Gowda said.
A very recent electron microscope study of a single nanowire has been performed by another scientist. If you want to see how a nanowire looks like when it gets charged with lithium ions, read our earlier article.
































